COVID-19 antigen rapid test kits for self-testing to be 'sold by pharmacists' to the public from Jun 16: MOH
A COVID-19 self-test kit. (Photo: AFP/Fred Tanneau)
SINGAPORE: COVID-19 antigen rapid test (ART) kits for self-testing will be "sold by pharmacists" to the public from Jun 16, said the Ministry of Health (MOH) on Thursday (Jun 10).
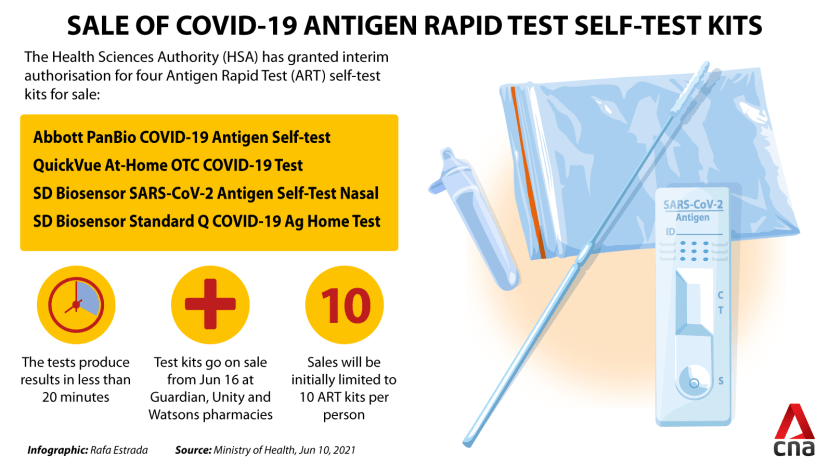
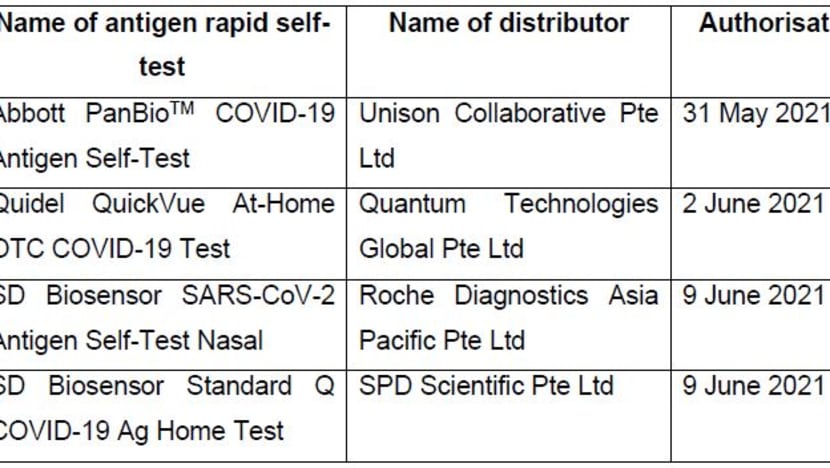
These self-test kits have received interim authorisation from the Health Sciences Authority (HSA) to be sold to the general public. The kits are: Abbott PanBio COVID-19 Antigen Self-test, QuickVue At-Home OTC COVID-19 Test, SD Biosensor SARS-CoV-2 Antigen Self-Test Nasal, and SD Biosensor Standard Q COVID-19 Ag Home Test.
These tests produce results in less than 20 minutes, said the Health Ministry.
READ: 4 new community COVID-19 cases in Singapore, including 2 unlinked
READ: Singapore must test 'faster, more liberally and extensively'; DIY tests soon available over the counter, says PM Lee
"They are simple to use, they can be self administered. From next week, Jun 16, these kits will be dispensed by pharmacists at selected retail pharmacies. We will then open up for counter sales at more retail locations progressively," said Health Minister Ong Ye Kung at a multi-ministry task force press conference.
Sales will initially be limited to 10 ART kits per person to ensure that there are "adequate supplies for all”, said the Health Ministry's director of medical services Kenneth Mak.
But as more supplies are made available for retail sales, authorities will "eventually allow test kits to be freely purchased", he said.
READ: Up to 5 in a group allowed from Jun 14; dining-in may resume on Jun 21 in phased easing of COVID-19 curbs
Singapore is set to ease some COVID-19 measures from Jun 14 as the number of community cases falls.
"As we want to resume more activities, we need to make testing fast, easy and accessible," said Mr Ong.
Associate Professor Mak added that the self-test kits “complement” Singapore’s overall surveillance strategy.
"These fast and easy to use tests allow us to detect infected cases more quickly, in particular among individuals who do not have acute respiratory infection symptoms, but are concerned that they may have been exposed to COVID-19," he said.
READ: Singaporeans aged 12 to 39 can register for COVID-19 vaccination from Jun 11
People who have a positive result on these self-test kits should "immediately approach" a Swab and Send Home Public Health Preparedness Clinic (SASH PHPC) for a confirmatory polymerase chain reaction (PCR) test, said MOH.
They are then required to self-isolate until they receive a negative PCR test result, MOH added.
Those who test negative on their self-test ART should continue to stay vigilant and adhere to prevailing safe management measures.
“Individuals who have ARI symptoms should continue to visit a doctor for a full diagnosis and PCR test instead of relying on an ART self-test kit,” the ministry said.
More information on how the self-test kits are to be used and how the results can be interpreted will be made available through various media channels and the MOH website from Jun 16, said Assoc Prof Mak.
CNA has contacted MOH for more information about the price of the self-test kits.
READ: Singapore eases COVID-19 restrictions in two phases: What is allowed and when
READ: Working from home to remain as default; Jobs Support Scheme extended for certain sectors
ARTS HAVE HIGHER CHANCE OF FALSE NEGATIVE RESULTS: HSA
In a separate press release on Thursday, the HSA said that the self-test kits can be bought without a doctor's prescription.
ARTs detect the viral proteins in the nasal swab samples of infected people and "usually work best in the early stages of infection", said the authority.
"In general, ARTs can achieve a sensitivity of about 80 per cent for cases with higher viral loads and a specificity range of 97 to 100 per cent," said the HSA.
Sensitivity refers to the test’s ability to correctly detect COVID-19 in individuals with the disease, while specificity refers to the test’s ability to correctly identify individuals without COVID-19, explained the authority.
ARTs have lower sensitivity than PCR tests, which means the rapid tests have a "higher chance of false negative results".
READ: COVID-19 vaccination: Why some seniors are holding back, and how a little nudge can help
Incorrect sample preparation or testing process when using the test, or a low viral protein level in the user’s nasal sample – for example, one to two days after potential exposure to the virus – could also result in a false negative result, HSA added.
All kits will come with leaflets providing instructions for use, and HSA urged consumers to read these carefully before using the tests.
"Users should collect their nasal sample using the swab provided in the kit and prepare their nasal sample using the buffer and tube provided.
"Once the sample is ready, users should perform the test using the test device and read the results," said the authority, adding that people should follow the instructions in the leaflet closely in order to get valid results.
HSA also noted that as part of its interim authorisation, these test developers are required to collect the relevant accuracy and safety data and monitor the use of their tests.
Additional data from ongoing clinical studies must also be submitted post-approval for HSA to ensure the continued safety and efficacy of these tests, HSA said.
BOOKMARK THIS: Our comprehensive coverage of the COVID-19 pandemic and its developments
Download our app or subscribe to our Telegram channel for the latest updates on the coronavirus outbreak: https://cna.asia/telegram












