FAQ: What you need to know about the new DIY COVID-19 antigen rapid test kits
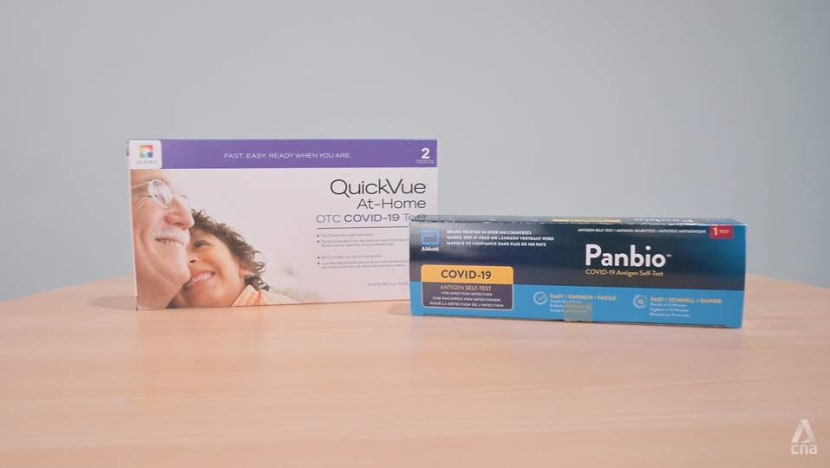
The Abbott PanBio COVID-19 Antigen Self-test and the QuickVue At-Home OTC COVID-19 Test are two of the four self-testing kits that have been given interim approval by HSA. (Photo: Gaya Chandramohan)
SINGAPORE: COVID-19 antigen rapid test (ART) kits for self-testing will be dispensed in pharmacies to the public from Wednesday (Jun 16), the Ministry of Health (MOH) announced on Jun 10.
ARTs detect the viral proteins in the nasal swab samples of infected individuals and usually work best in the early stages of infection.
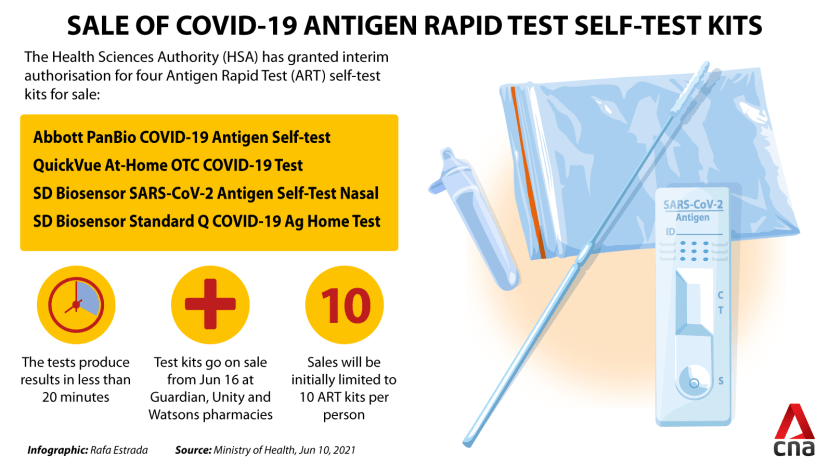
Four self-test kits have received interim authorisation from the Health Sciences Authority (HSA) to be sold to the general public: The Abbott PanBio COVID-19 Antigen Self-test, QuickVue At-Home OTC COVID-19 Test, SD Biosensor SARS-CoV-2 Antigen Self-Test Nasal, and SD Biosensor Standard Q COVID-19 Ag Home Test.
READ: COVID-19 antigen rapid test kits for self-testing to be 'sold by pharmacists' to the public from Jun 16: MOH
According to the Health Ministry, these tests produce results in less than 20 minutes.
If you’re planning to pick some up when they go on sale, here’s what you need to know about these self-test kits.
A: The kits will be available at selected Unity, Watsons and Guardian outlets islandwide.
From Jun 16, these kits will be dispensed by pharmacists at selected retail pharmacies, said Health Minister Ong Ye Kung on Jun 10.
“We will then open up for counter sales at more retail locations progressively," he added.
The test kits will be dispensed by the pharmacist in-store, which means customers have to consult a pharmacist before purchasing them. They can be bought without a doctor’s prescription, said HSA in an update on Jun 10.
According to Quantum Technologies Global, the distributor of the QuickVue tests, training will be provided to pharmacists on how to teach customers on the correct usage of the tests.

All 79 Guardian stores with an in-store pharmacy will carry the COVID-19 ART kits including the Guardian store at the Giant outlet in Suntec City, said a Dairy Farm Group spokesperson in response to CNA's queries.
Customers can visit Guardian’s website to use the "Pharmacy" filter to locate these stores.
The Abbott PanBioTM COVID-19 Antigen Self-test and the QuickVue At-Home OTC COVID-19 Test will be available at Guardian outlets, the spokesperson added.

39 Unity pharmacies will offer the test kits from Jun 16, said a FairPrice spokesperson in response to CNA's queries.
These stores were “specially selected” as they have in-store pharmacists who are “professionally trained” to assess whether customers are suitable to use the ART kits and provide information on how to use them, said the spokesperson.
The Abbot Panbio COVID-19 Antigen Self-Test and Quidel QuickVue At-Home OTC COVID-19 Test kits will be available at all Watsons pharmacy stores in the first phase of the test kit roll out, said a spokesperson for the company.
The self-test kits will be gradually expanded to more Watsons stores and Watsons Online in the second phase, the spokesperson said in response to CNA queries.
Consumers will be able to locate Watsons pharmacy stores using the store finder option on the company’s website or via the store locator on the Watsons SG mobile app.
Q: How much will the test kits cost and how many can I buy?
Sales will initially be limited to 10 ART kits per person to ensure that there are "adequate supplies for all”, said the Health Ministry's director of medical services Kenneth Mak on Jun 10.
But as more supplies are made available for retail sales, authorities will "eventually allow test kits to be freely purchased", he said.
According to Watsons, the pharmacy will be keeping with MOH’s recommended guidelines for the selling price of the kits. This ranges from S$10 to S$13 per test kit depending on the size of the pack purchased, said the spokesperson.
“We would advise the public to keep to the guidelines of maximum 10 test kits per customer so as to ensure there would be sufficient test kits for everyone. We would be monitoring the demands closely and stock up to meet with the consumer needs,” the spokesperson added.
The FairPrice spokesperson said that details on the type of kits and pricing are still being finalised, and more information will be available soon.
Dairy Farm's spokesperson also said the company will adhere to MOH's pricing guidelines.
CNA has contacted the ministry for more information about the price of the self-test kits.
From Jun 16, Quantum Technologies Global will have about 500,000 tests available, and more kits will be flown in from the United States in the coming weeks, said a company spokesperson in response to CNA queries.
Abbott is “well-positioned” to fulfill the demand for COVID-19 tests, said Abbott’s APAC divisional vice-president for rapid diagnostics Sanjeev Johar.
“We expect to make millions of Panbio Antigen Rapid Tests available for Singapore over the coming months, as needed,” he added.

Q: How do these test kits work? What should I look out for?
Those using the self-test kits should collect their nasal sample using the swabs provided in the kits, said HSA in the press release on Jun 10.
They should then prepare their nasal sample with the buffer and tube provided. Once the sample is ready, users should use it with the test device and read the results, said HSA.
While performing the test, users should follow the instructions in the instruction leaflets to get valid results, the authorities said.
The instructions for all four self-test kits may differ slightly. For example, the QuickVue test uses a test strip dipped in the buffer solution, while the one manufactured by Abbott involves dripping the buffer solution on a rapid test device.
"For children younger than 14 years, an adult caretaker should help collect the nasal samples and conduct the test procedures," said Abbott.

Q: Are these test kits effective and accurate? What should I do if I test positive?
In general, ARTs can achieve a sensitivity of about 80 per cent for cases with higher viral loads and a specificity range of 97 to 100 per cent, said HSA.
Sensitivity refers to the test’s ability to correctly detect COVID-19 in individuals with the , while specificity refers to the test’s ability to correctly identify individuals without COVID-19.
ARTs have lower sensitivity than the polymerase chain reaction (PCR) tests, which means such tests have a "higher chance of false negative results", said HSA in the press release.
Incorrect sample preparation or testing process when using the test, or a low viral protein level in the user’s nasal sample – for example, one to two days after potential exposure to the virus – could also result in a false negative result, HSA added.
Infectious diseases expert Dr Leong Hoe Nam urged users to follow the instructions on how to use the test kits strictly and “to the letter”.
A properly done test will “carry a similar sensitivity” to a PCR test, especially if it’s repeated every three to five days, he added.
“A negative test doesn’t mean you don’t have it but you’re less likely to have COVID-19,” said Dr Leong.

Q: What should I do if I see a positive result?
People who have a positive result on these self-test kits should "immediately approach" a Swab and Send Home Public Health Preparedness Clinic (SASH PHPC) for a confirmatory PCR test, said MOH.
After that, they must self-isolate until they receive a negative PCR test result, MOH added.

Those who test negative on their self-test ART kit should continue to stay vigilant and adhere to prevailing safe management measures, said the Health Ministry.
“Individuals who have ARI symptoms should continue to visit a doctor for a full diagnosis and PCR test instead of relying on an ART self-test kit.”
BOOKMARK THIS: Our comprehensive coverage of the COVID-19 pandemic and its developments
Download our app or subscribe to our Telegram channel for the latest updates on the coronavirus outbreak: https://cna.asia/telegram












