Commentary: How do we justify using animals as organ donors for humans?
If it is ever ethically justified to sacrifice animals to benefit humans, it would be to save an identifiable human life. But the interests of animals should be given appropriate weight in xenotransplantation decisions, say two NUS researchers.

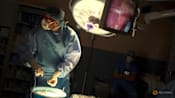
File photo. This handout photo shows surgeons performing a transplant of a heart from a genetically modified pig to patient David Bennett, Sr. (Photo: AFP/University of Maryland School of Medicine)
SINGAPORE: In January this year, David Bennett, a 57-year-old patient in the US, became the first person in the world to be transplanted with the heart of a genetically modified pig. He was certain to die from terminal heart disease and was not medically eligible to receive a human heart from a deceased donor or a mechanical heart pump.
The experimental treatment held important implications for transplant medicine, as it showed that it was feasible to transplant pig organs into humans.
Unfortunately, Bennett died two months later. A virus called porcine cytomegalovirus was found in the pig heart, which is believed to have contributed to his death.
Historically, there have been many attempts at transplanting living non-human biological materials into human recipients for therapeutic purpose, otherwise known as xenotransplantation.
HOW ANIMAL ORGANS MADE THEIR WAY INTO HUMANS
Experimental transplantation of solid organs such as hearts, kidneys and liver from a variety of animals into human patients started in the 1960s, prior to the institutionalisation of human organ donation systems. Most patients who received solid organ xenografts died days or weeks after because of immunological rejection or an infection complication.
Advances in science and technology could remove or reduce these barriers to xenotransplantation. To prevent rejection, animals purposed for xenotransplantation could be genetically modified by genome editing to have human-like immune systems. Viruses that commonly infect animals, such as porcine cytomegalovirus in pigs, could be prevented from infecting human recipients through pathogen-free breeding.
Pathogen-free breeding would not prevent all cross-species infection as some viruses are native in animal cells because of certain genes, which could pass on to human cells and mutate to cause conditions like cancer. But genome editing could “knock out” these virus genes and reduce the risk of transmission.
More than 100,000 in the US alone are waiting for a life-saving organ transplant, and about 17 people there die each day from this wait.
WILL SINGAPORE SEE ANIMAL-TO-HUMAN TRANSPLANTS?
Like other countries, Singapore faces a shortage of human kidneys and other transplant organs or tissue. The average waiting time for a kidney transplant is about 8.1 years in Singapore, followed by 3.2 years for a heart.
There were 541 deceased and living donor organ transplantations (kidney, liver, heart, cornea, lung and pancreas) in 2021, compared with 605 transplants for these organs in 2019 before the pandemic hit.
As of June, there were 604 patients on the waiting list for a transplant.
Amid an ageing population, could Singapore consider animal-to-human organ transplants to address the shortage?
Xenotransplantation trials could be conducted in Singapore to study its safety, effectiveness, and public health risks, especially if suitable pigs could be sourced safely from overseas and there is sufficient data on its safety and efficacy in other countries to support these trials.
If xenotransplantation were to be conducted locally as clinical trials, there is a need to look at the permissibility and feasibility of xenotransplantation procedures under existing laws.
This would mean reviewing current law, regulations and guidelines governing clinical trials or biomedical research and use of animals for scientific purposes, and guidance from the Health Sciences Authority of Singapore, which classifies “xeno-based products as “higher risk” therapeutic products.
Other international standards should also be considered in deciding the appropriate limits. The World Health Organization states that member states should allow xenotransplantation only when effective national regulatory control and surveillance mechanisms are in place.
Any xenotransplantation trial in Singapore would need to be approved by an institutional review board, which provides ethical oversight of research activities by weighing their risks and benefits and reviewing informed consent forms and processes for potential patient participants. Other ethics committees such as transplant ethics committees would likely also provide ethical oversight.
As xenotransplantation raises a unique set of ethical considerations and concerns, it may be beneficial to establish a separate, new interdisciplinary committee consisting of transplantation and veterinary professionals and those who have been involved in transplantation ethics, animal research ethics, as well as clinical and research ethics review.
POSSIBLE FOR XENOTRANSPLANTATION TO BECOME STANDARD TREATMENT?
By using genetically modified animals to ensure immunological acceptance and improving virological safety measures, xenotransplantation could help address the increasing severe shortage of donated human organs, and ethical issues like how to fairly exclude patients from waiting lists or fairly allocate the limited human organs among those waiting.
Besides patients with end-stage organ failure, transplantation of animal cells and tissues could help other patients, such as those with burn wounds, corneal blindness, and neurological disorders, where human biological materials are not available.
But xenotransplantation also raises many scientific and clinical questions that must be answered before it becomes standard treatment similar to human-to-human organ transplant.
How long can a person live with the heart of an animal? Would an animal organ function as well as a human organ? What is the best mix of animal genetic modification and immunosuppressive drugs to ensure short- and long-term graft acceptance, given a recipient’s condition? What type and which breed of animal is most suitable for providing transplant organs for humans?
Domestic pigs are currently the animal of choice for kidney or heart xenotransplantation because their hearts and kidneys are similar in size and function to us.
Recently, in separate experiments, transplant surgeons in the US successfully transplanted genetically modified pig kidneys and hearts in brain-dead patients on life support (with their or their family’s prior consent) to monitor graft rejection, survival and function. These experiments are an intermediate step to collect data on the clinical safety and efficacy of pig-to-human organ transplant without risking the life of a patient.
TRIALS HAVE TO INVOLVE PATIENTS THAT ARE ALIVE
Ultimately, transplant surgeons have to conduct clinical trials on living human patients as they are the only way to prove the safety and effectiveness of xenotransplantation.
These trials raise various questions of their own: Do we limit participation to critically ill patients who cannot wait for a human donor? Post-xenotransplantation, do participants need to undergo lifelong virus monitoring, which could include the need to provide tissue sample for laboratory analysis?
Bioethicists have argued for the need for lifelong monitoring based on the need to prevent harm to others. Animal viruses integrated into animal genomes could stay latent for many years before they cause disease (and become detectable), and they could subsequently transmit to the close contacts of xenograft recipients and the wider human population.
Participants in xenotransplantation trials could be unlike participants in other types of clinical trials, as they may have to waive their right to withdraw from research (or at least from monitoring as a public health safety measure), which is a central tenet in research ethics.
IS XENOTRANSPLANTATION A FORM OF ANIMAL ABUSE?
Xenotransplantation is an area of controversy. Despite the uncertainties, it invites us to think about the way we relate to animals and use them for our benefit. Animal rights activists and ethicists have opposed xenotransplantation as another form of animal abuse.
If it is ever ethically justified to sacrifice animals to benefit humans, it would be to save an identifiable human life. The interests of animals, which could feel pain and suffering like us, should be given appropriate weight in xenotransplantation decisions.
Consideration should be given to whether human recipients who are unlikely to survive for a meaningful period of time even with a xenograft or who are unlikely to comply with post-transplant advice and care, should be excluded from xenotransplantation trials so as to prevent the unnecessary sacrifice of animals.
Public engagement would be vital to help health policy makers understand the public acceptability and limits of xenotransplantation in a society.
Dr Voo Teck Chuan is an Assistant Professor and Dr Chan Hui Yun is a Research Fellow at the NUS Centre for Biomedical Ethics, Yong Loo Lin School of Medicine.