Novavax's COVID-19 vaccine to be made available to adolescents aged 12 to 17
From May 15, individuals in that age group may book an appointment to receive the vaccine at any of the 19 Public Health Preparedness Clinics offering it.
File photo of a healthcare worker preparing a dose of Nuvaxovid COVID-19 vaccine. (Photo: Jeroen Jumelet/ANP/AFP)
SINGAPORE: The Novavax COVID-19 vaccine will be offered to adolescents aged 12 to 17 from May 15, the Ministry of Health (MOH) announced on Friday (May 12).
MOH said it accepted the recommendations from the Expert Committee on COVID-19 Vaccination to extend the use of the vaccine to this age group under the National Vaccination Programme.
This follows the Health Sciences Authority's authorisation of the vaccine for use in this age group.
With this extension, individuals in this age group who are medically ineligible to receive the Pfizer-BioNTech/Comirnaty vaccine, or who wish to receive a non-mRNA vaccine, will have the option of receiving a non-mRNA jab under the programme to complete their recommended COVID-19 vaccinations.
The Novavax COVID-19 vaccine, also known as Nuvaxovid, was granted interim authorisation in Singapore on Feb 14, 2022.
From May 15, adolescents aged 12 to 17 may book an appointment to receive the vaccine at any of the 19 Public Health Preparedness Clinics offering it.
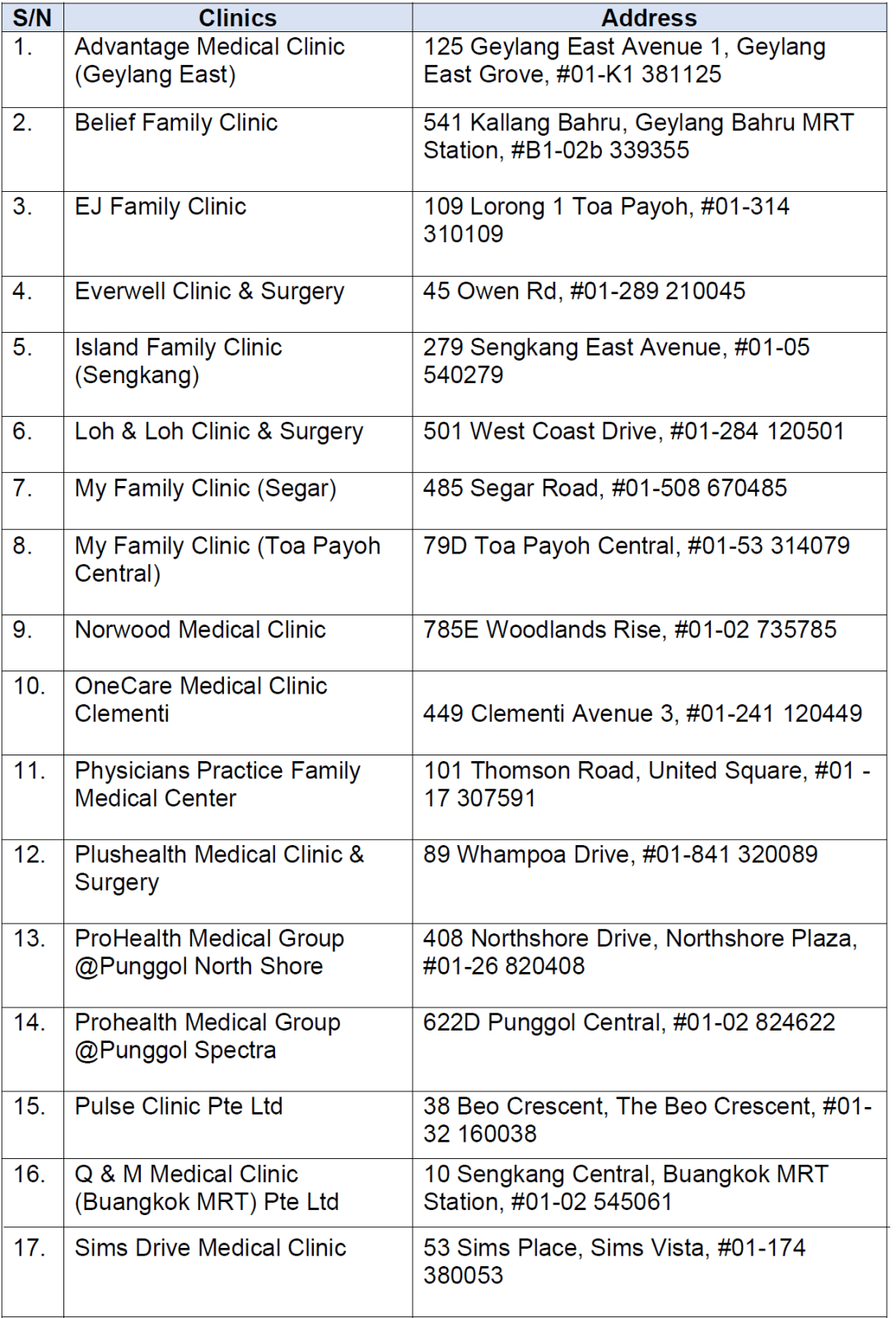
They can also visit gowhere.gov.sg/vaccine to locate the nearest vaccination site and their operating hours.
Adolescents aged 12 to 17 taking the Novavax COVID-19 jabs are recommended to have three doses of the vaccine to achieve minimum protection, said MOH.
The first and second doses are to be taken eight weeks apart, while the recommended interval between the second and third doses is five months. The Novavax vaccine may be taken as a booster jab even if a person had previously only received mRNA vaccines.
According to MOH, those aged five and above should complete three mRNA or Novavax vaccine doses, or four doses of the Sinovac-CoronaVac vaccine to achieve minimum protection against COVID-19,
In a separate statement, the expert committee said the Novavax vaccine generated comparable antibody levels in adolescents aged 12 to 17 as it did in adults. It also had a vaccine efficacy of 79.5 per cent while the Delta variant was circulating.
Most of its side effects were mild to moderate in severity and resolved within days. No local cases of myocarditis, or inflammation of the heart, have been observed. However, very rare reports of myocarditis were reported overseas.
The committee added that the Novavax vaccine is not an updated vaccine, unlike the Pfizer-BioNTech/Comirnaty Bivalent vaccine available to the 12 to 17 age group under the National Vaccination Programme.
"Vaccination remains our primary defence against COVID-19. We encourage all eligible individuals to complete the vaccine doses, including any boosters, recommended for them so as to minimise the risk of developing severe illness," said MOH.
Other places that have approved the Novavax vaccine for adolescents aged 12 to 17 include the United States, Australia, Taiwan and South Korea.