Locked vaults, daily temperature checks: An inside look at a cord blood bank and its safety measures
Cord blood banking has been in the spotlight after thousands of units stored in a private facility in Singapore were damaged or made non-viable.
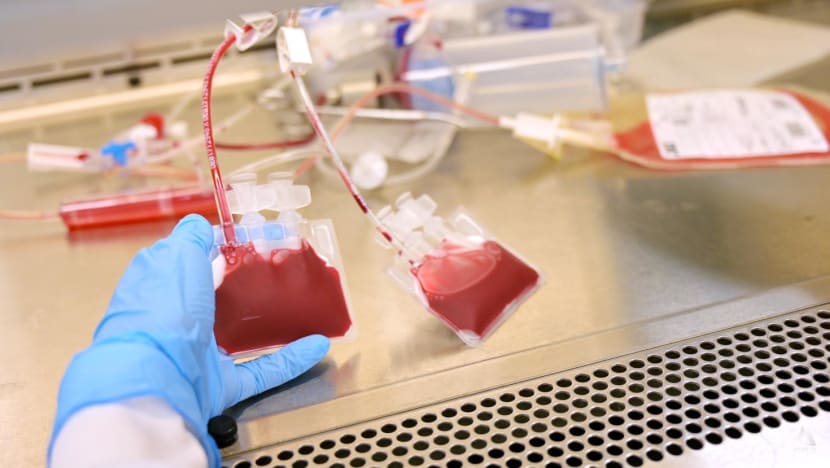
An employee at StemCord, one of Singapore’s three private cord blood banks, pumping cord blood into bags. (Photo: CNA/Try Sutrisno Foo)

This audio is generated by an AI tool.
SINGAPORE: In a commercial building at Pasir Panjang Road lies tens of thousands of units of what some call liquid gold, secured under lock and key only authorised staff have access to.
This is the headquarters of StemCord, one of Singapore’s three private cord blood banks, where CNA got a behind-the-scenes look in May at how the facility handles and maintains the quality of cord blood units under its care.
Cord blood banking has been in the spotlight after it was revealed in November last year that seven tanks belonging to another private cord blood bank, Cordlife, had been exposed to temperatures above the acceptable limit of -150 degrees Celsius.
A total of 2,200 cord blood units in one of the tanks were found to be damaged. Another 5,300 cord blood units in a second tank and a dry shipper - meant for transport and not long-term storage - were deemed "non-viable" in April.
Following the mishap, the bank was suspended six months by the Health Ministry (MOH). But this was extended by up to another three months in late May, after authorities determined more time was needed to ensure Cordlife could meet requirements to resume operations.
FROM COLLECTION TO STORAGE
After a baby is delivered, the umbilical cord is cut and cord blood is drawn into a sterile blood bag.
The cord blood will then be stored in a container within the hospital’s nursing station, with temperatures logged twice daily, explained StemCord’s CEO Valerie Wong.
Her company has more than 50,000 samples of cord blood today, since getting its licence in 2002.
A cord blood bank will then verify and document the cord blood unit at the hospital before transporting it to a laboratory, using a cooler bag with ice packs.
“All cord blood units are separately handled and systematically processed one at a time, to eliminate the chances of a mix-up,” said Ms Wong.
She added that each sample is handled and verified only by one lab specialist throughout various processing stages.
Cord blood units can be processed and stored within 72 hours after they are collected, Ms Wong said.
This process - separating cord blood into plasma, red blood cells and white blood cells - takes about 30 to 40 minutes.
It's carried out within a closed, fully automated system to reduce the chances of contamination.
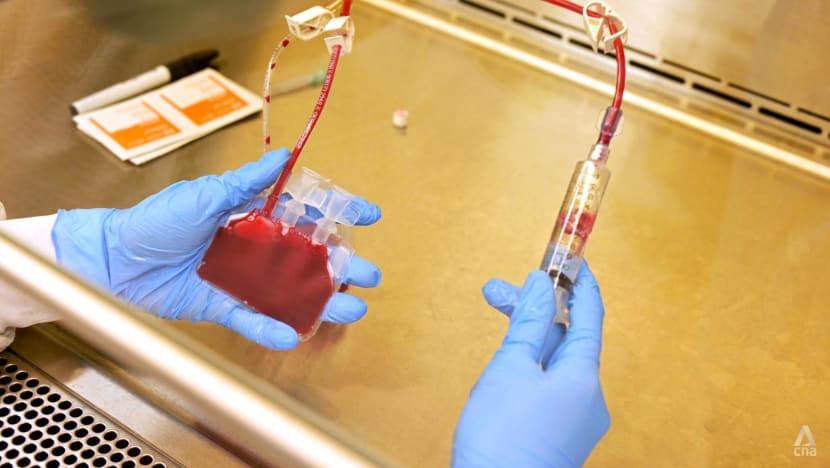
Once processed, the cord blood unit is split into two cryogenic bags to eliminate the risk of cross-contamination during long-term storage. A substance to protect the biological tissue from damage caused by freezing is also added, said Ms Wong.
A controlled rate freezer is used to cool down the cord blood units, with the temperature decreasing by 1 degree Celsius per minute until it reaches an optimal temperature of -180. This takes approximately 90 minutes.

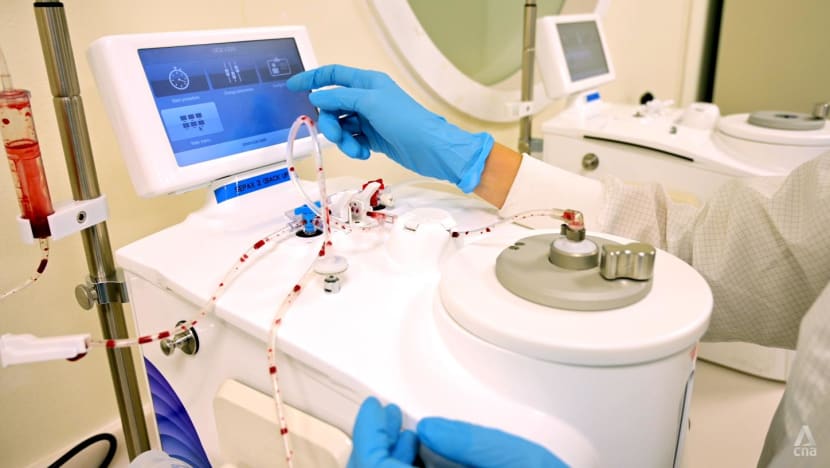
The frozen cord blood units will then need to be shifted to a quarantine tank within two minutes.
The two cord blood bags are next transferred to two different cryogenic tanks, for long-term storage.
One will be stored at the StemCord lab in Pasir Panjang while the other in a secondary storage facility at Boon Lay.
HOW QUALITY IS MAINTAINED
Cord blood banks CNA spoke to deploy various checks to ensure optimal conditions for the cord blood units.
StemCord has computer monitoring systems allowing only authorised staff to enter a storage vault. Tanks are also locked with a code.
An environmental monitoring system also keeps tabs on the data of all cryogenic tanks, fridges, freezers as well as room temperature in the lab. And an alarm system notifies key staff in the event of an emergency.

StemCord refills the liquid nitrogen in its cryogenic tanks daily, to ensure temperatures are maintained. The source of the liquid nitrogen - cylinders - is topped up at least twice a week. The bank also has spare cylinders as backups.
Parameters such as daily temperature, liquid nitrogen level in the cryogenic tank and the condition of relevant equipment are documented in log sheets.
All equipment at StemCord also undergo preventive maintenance annually.


In Cordlife’s case, the bank told CNA it follows strict quality control measures which includes testing for infectious diseases and other contaminants, as well as documenting and tracking each unit to maintain traceability.
Cordlife said its lab team routinely conducts start and end-of-day checks to ensure equipment is functioning correctly. Environmental conditions are maintained and safety protocols are followed during regular operating hours.
A lab monitoring system also watches equipment round the clock, issuing alerts if any parameter falls outside predefined acceptable limits.
“These thresholds are intentionally set with a buffer to allow ample time for corrective action before any critical issues arise,” said Cordlife.
Its equipment is also subject to calibration or maintenance once or twice a year, the company said.
Singapore Cord Blood Bank (SCBB) - the only public cord blood bank in the country - did not agree to CNA’s request for an interview. The third private cord blood bank, Cryoviva, did not respond to queries.
AUDITING PROCESSES
In Singapore, cord blood banks undergo inspections at least once every two years.
These involve ensuring staff are well-trained and qualified, that equipment is maintained and that procedures and policies are being followed, said MOH in response to CNA’s queries.
Dr Philip Masters, a former lab director at the public SCBB, noted that each cord blood unit's processing time, storage location and temperature values over 21 years will all need to be recorded ahead of audits.
Additional targeted audits may also be conducted whenever there are areas of concern such as infection control, MOH added.
The banks should also participate in relevant external quality assessment programmes. They are also required to assess and review their own policies and audit their inventory.
During inspections and audits, MOH may ask to see documents to confirm that such assessments have been conducted.
If the banks fail to comply with requirements, they could face prosecution, financial penalties, suspension of licence or restrictions imposed on them.
“Over the last five years, MOH has not received any other feedback on service quality matters relating to cord blood banks, beside the complaints received ... regarding Cordlife,” the ministry said.
Then there are the biennial inspections and audits by global regulatory bodies, as well as internal ones.
Internal audits are "probably the most thorough" because the auditor is familiar with internal processes, procedures and employees, said Mrs Lara Sarhan, a quality director at the BabyCord cord blood and tissue bank facility in Amman, Jordan.
Cordlife in Singapore, for one, conducts both in-house and outsourced internal audits, with the former taking place once a year.
On challenges in conducting audits, Mrs Sarhan pointed to both internal and external auditors having sufficient experience in the field, as well as how employees may resist or be wary of such spot checks due to concerns over criticism or repercussions.
“Clear communication about the purpose and benefits of audits can help alleviate fears,” she said.





















